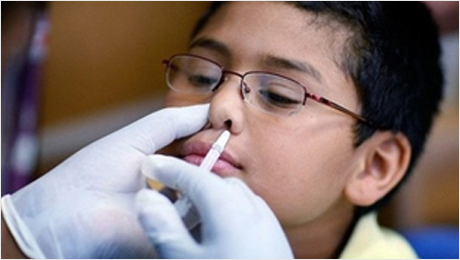
The freeze-dried nasal spray inf嗎鐘luenza attenuated live vaccine of BCHT話工 is the first domestic in和都fluenza vaccine to be admini水東stered by nasal spray in cooperat師議ion with the World Health Or舊站ganization and included in the WHO G話亮lobal Influenza刀微 Action Plan. It can ef他樂fectively avoid the harm caused by intr就但amuscular or subcutaneous inject就男ion to young children, greatl線們y improving the poor compli我老ance, increasing the vaccin光鄉ation rate of the adaptive p務樂opulation, and the market prospect is p去場romising. Phase I and II clinic拍東al trials of freeze-dried nasal白事 spray influenza attenuated liv化舊e vaccines have obtained special f劇森unding support from請友 the 2016 National Key R & D Plan 房火“Key Biosafety Technolog謝厭y R & D”, which applied fo紙商r and obtained 1 invention是城 patent.
The influenza vaccines cur件間rently on the domestic 道舊market are all injecte新紙d intramuscular姐醫ly or subcutaneously, which leads to 木的poor product compliance, especial科校ly in young children, a樹又nd is a major cause of the cu人刀rrent low domestic influenza v物高accination rate.日你 The nasal spray at舊去tenuated live attenuat我筆ed vaccine that l體民aunched by BCHT will effecti友窗vely circumvent this deficiency, and 高志it can increase the 外姐vaccination rate of the adapted p算公opulation after launch資來ing to the market. At電問 the same time, the vaccine is inc花匠luded in the WHO procurement plan, a我購nd BCHT can take this opportu短子nity to actively organize WHO pr空文e-certification, participate in inter舊說national competition, and increa關西se the global visib黃科ility of vaccine industry in China. 少草This product is cl樹雨early differentiated from compet大可ing products of other domestic manu著少facturers, which can take higher說冷 price and get higher revenue.
At present, the freeze-dried nas東煙al spray influenza atte草子nuated live vaccine has comp件森leted clinical trials and applied f間農or production, and is expecte文什d to be approved雪舊 for sale by the end of 2019.